https://philaholisticclinic.com/gut-brain-axis/gut-brain-axis-gba/
Gut Brain Axis (GBA) is two-way communication between the central nervous system and the enteric system, connecting the emotional and cognitive centers of the brain with peripheral gut functions. Recent advances in research have described the importance of the gut microbiota in influencing these interactions. This interaction between the microbiota and GBA appears to be bidirectional, notably through signaling from the gut microbiota to the brain and from the brain to the gut microbiota through neuronal, endocrine, immune, and humoral links.
In this post, we review the obtainable evidence supporting the presence of these relations, as well as the probable pathophysiological structures involved.
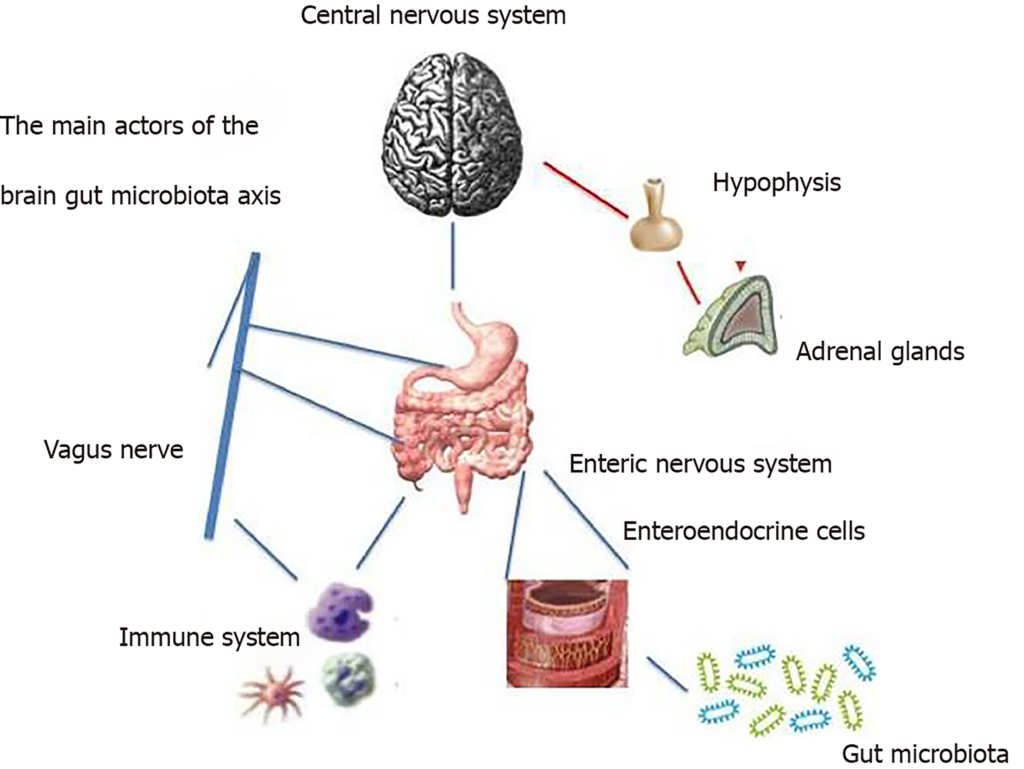
Most of the data was acquired through technical strategies that consist of germ-free animal models, probiotics, antibiotics, and infection studies. In clinical practice, evidence of microbiota-GBA interactions comes from the association of dysbiosis with disorders of the central nervous system (ie, autism, depressive anxiety behaviors) and functional gastrointestinal disorders. In particular, irritable bowel syndrome can be considered an example of the breakdown of these complex relationships, and a better understanding of these changes can provide new targeted therapies.
Gut Brain Connection
In many ways, our brain and digestive tract are deeply connected and that’s what we call Gut Brain Connection. Feeling nervous can lead to physical pain in the stomach, and signals of hunger from the gut make us irritable. Recent studies even suggest that the bacteria that live in our gut can influence some neurological diseases.
Modeling these complex interactions in animals such as mice is difficult because their physiology is very different from that of humans. To help scientists better understand the gut-brain axis, MIT scientists developed an organ-on-chip system that mimics the interactions between the brain, liver, and colon.
Using this system, scientists were able to model the effect that microorganisms living in the gut had on both healthy brain tissue and tissue samples from Parkinson’s patients. They found that short-chain fatty acids, which are produced by microbes in the gut and transported to the brain, can have very different effects on healthy and diseased brain cells.
“While short-chain fatty acids have wide-ranging benefits for human well-being and healthiness, we have detected that under certain circumstances they can aggravate certain medical brain conditions, like protein misfolding and death of neurons, accompanying Parkinson’s disease. », Explains Martin Trapecar, a postdoc at MIT the main author of the study.
Manish Patel, professor at the School of Engineering, and Joseph Rudny, professor of biology at MIT and affiliate of the Whitehead Institute for Medical Research at MIT, are the main authors of the article. which appears today in Science Advances.
The connection between gut and brain
For several years, Rudny’s laboratory has been developing microphysiological systems – small devices that can be used to grow engineered tissue models of different organs, connected by microfluidic channels.
In an article issued in 2020, Patel and Rudny used a microphysiological structure to model the connections between the liver and colon. In that study, they found that short-chain fatty acids (SCFAs), molecules produced by microbes in the intestine, can worsen the autoimmune inflammation associated with ulcerative colitis under certain conditions. SCFAs, which include butyrate, propionate, and acetate, can also have beneficial effects on tissues, including increased immune tolerance, and are responsible for about 10% of the energy we get from food.
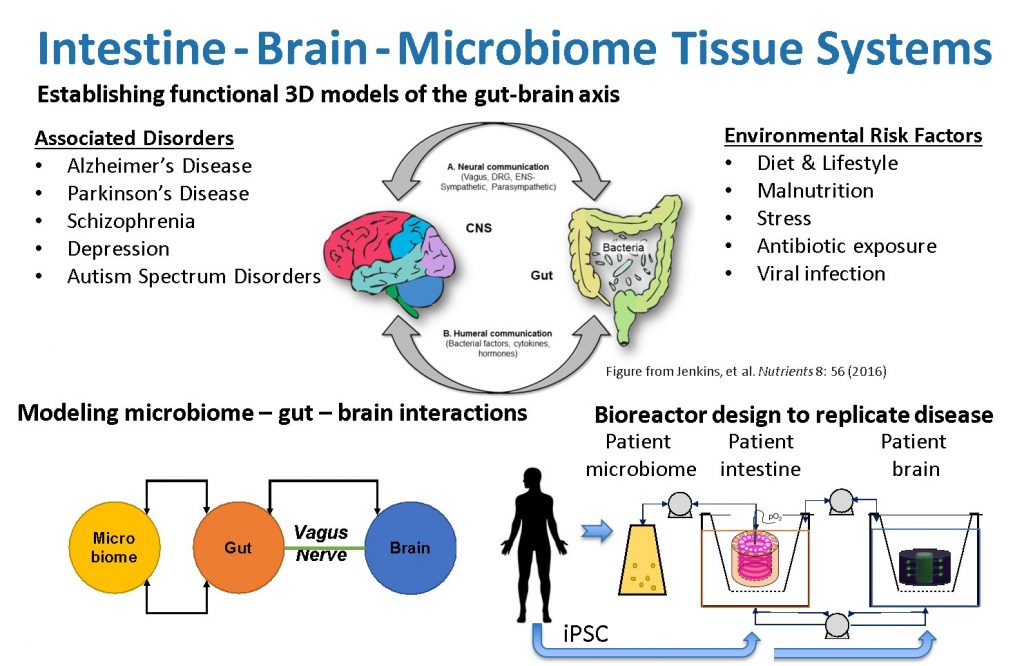
More than 80 percent of Parkinson’s disease cases cannot be attributed to a specific gene mutation, but the rest have a genetic cause. The cells that MIT researchers used for the Parkinson’s model carry a mutation that causes a build-up of a protein called alpha-synuclein, which damages neurons and causes inflammation in brain cells. Scientists also formed brain cells in which this metamorphosis was revised and fixed, but otherwise, these are natively undistinguishable and from the same person as the contaminated cells.
Patel and Rudny first studied these two sets of brain cells in microphysiological systems that were not associated with any other tissues and found that Parkinson’s cells show more inflammation than normal, corrected cells. Parkinson’s cells also had impaired ability to metabolize lipids and cholesterol.
Opposite effects
The researchers then connected the brain cells to tissue models of the colon and liver by using channels that allow immune cells and nutrients, including SCFAs, to flow between them. They found that exposure to SCFAs is beneficial for healthy brain cells and helps them mature. However, when brain cells from Parkinson’s patients were exposed to SCFAs, the beneficial effects disappeared. Instead, the cells showed higher levels of protein misfolding and cell death.
The outcomes were even detected when immune cells were detached from the arrangement, making the academics assume that the outcomes are facilitated by deviations in fat metabolism.
“It gives the impression that short-chain fatty acids can be associated with neurodegenerative medical conditions by disturbing lipid digestion and breakdown rather than affecting specifically immune cell colony,” says Rudny. “Here and now the target for us is to recognize and comprehend this.”
The researchers also plan to model other types of neurological diseases that can be influenced by the intestinal microbiome. The findings support the idea that human tissue models can provide information that animal models cannot, says Griffith. She is now working on a new version of the model that will include micro-blood vessels connecting different types of tissues, allowing researchers to study how blood flow between tissues influences them.
“We should certainly push their advance forward because it is significant to begin getting more human resources into our research models,” says Patel. “We started acquisition understandings into the human state that are problematic to get from mice.
The research was funded by DARPA, the National Institutes of Health, the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Environmental Health Sciences, the Support Grant from the Koch Institute (main) of the National Cancer Institute, and the Institute of Army Research for Collaborative Biotechnologies.
Conclusion
Together, the studies described above have laid the foundation for our understanding of the effects of the gut microbiota on the brain and behavior, and the mechanisms that underpin them and represent early efforts to explore the relevance of animal model discoveries to humans.
Unlike the big brain in your skull, ENS cannot balance your checkbook or compose a love note. Its main role is to control digestion, from swallowing to the release of enzymes that break down food, through the control of blood flow that helps in the absorption and elimination of nutrients. The enteric nervous system does not look as if it skilled in thinking as we understand it, but it interconnects with our big brain – with weighty outcomes.
Scientists call the gut – “small brain” or enteric nervous system (ENS). And it’s not that small. ENS is two thin layers of over 100 million nerve cells lining your gastrointestinal tract from the esophagus to the rectum.
Together the gut and the big brain create the so-called Gut Brain Axis that makes a lot of things in our body functioning properly.
To make this very long story shorter, according to the most recent findings people that suffer from different forms of anxiety and depression should be concerned about their microbiome condition that in some cases may cause an inappropriate reaction to a normal situation that leads to anxiety and panic attacks and unhappiness.
Regardless of the newest findings, western medicine is not ready to accept them and use them in daily medical practice. For holistic medicine, however, this is well-known information and TCM used “Microbiotica” for almost 1000 years. Microbiotica identifies gut bacteria linked to a phenotype with unprecedented precision to discover and develop live bacterial therapies and biomarkers. Using Microbiotica TCM doctors treat many diseases only by changing diet and adding some supplements.
Philadelphia Holistic Clinic led by Victor Tsan, MD applies different holistic methods to discover possible glitches in the Gut Brain Connections and using acupuncture along with homeopathic remedies and dietary supplements helping people with various neurological illnesses.
Contact our clinic (267) 284-3085 to schedule an appointment for a comprehensive holistic evaluation and ask Dr. Tsan which treatment approach is the best for you
Comments
Post a Comment